
Next were lipids, which are long chains of hydrocarbons that store a lot of energy for our bodies, such as butter, oils, waxes, cholesterol, etc.

Third were proteins, which make up most of our bodies and speed up chemical reactions, depending on what kind of protein they are. Structural proteins make up most of the structures in the body, and enzymes are catalysts that speed up chemical reaction that happen in the body.

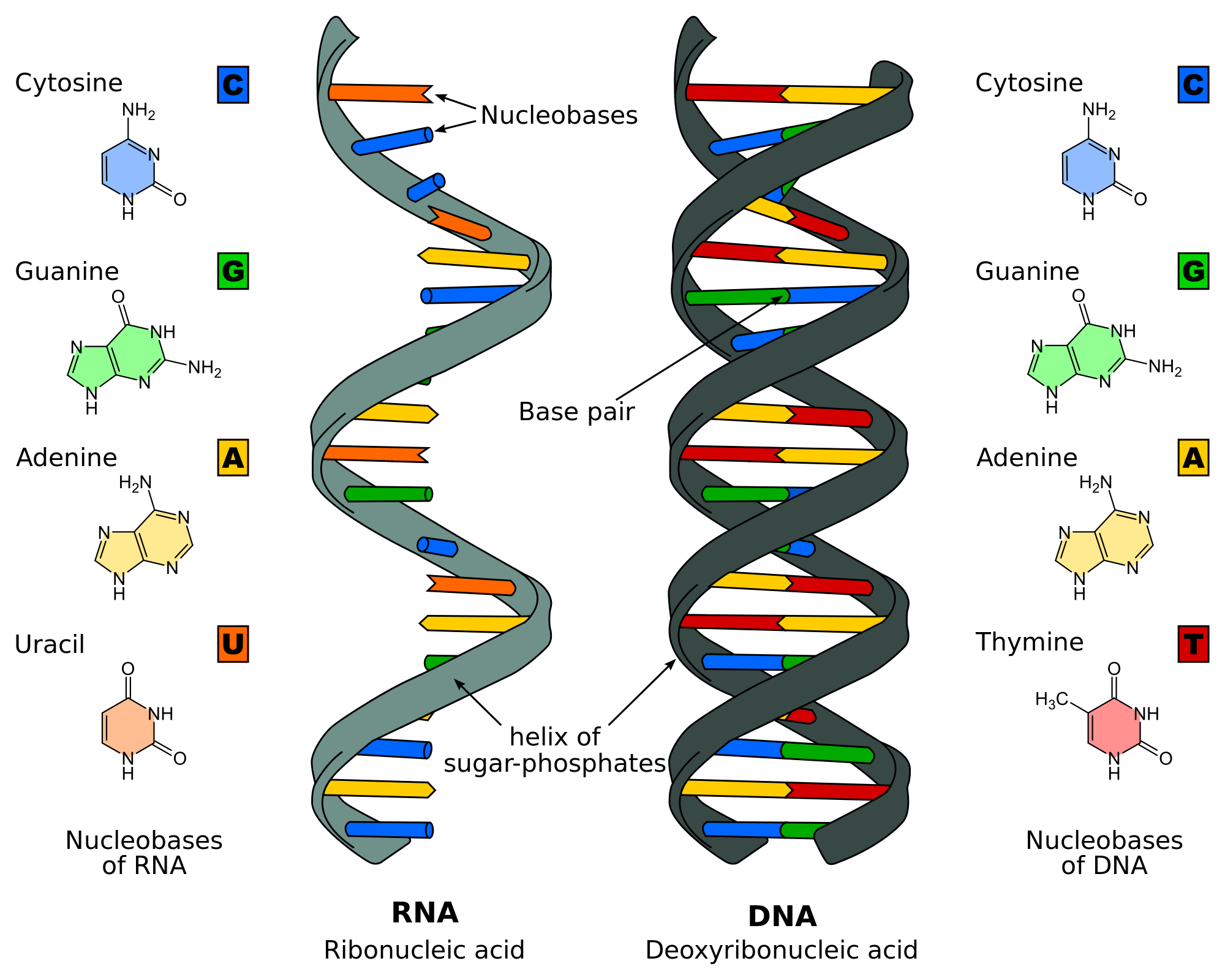
The last one we learned about were nucleic acids. Some nucleic acids that we learned about were DNA, RNA, and ATP. DNA is the blueprint for telling the body how to make proteins, RNA is a temporary copy of DNA that also tells the body how to make proteins, and ATP is the nucleic acid that transports energy throughout the body.
This unit went well in my opinion. Some of the things we learned were review for me, but it went more in depth than what I had learned in previous years. My strengths were that I had already known much of this knowledge from Life Science in 7th grade and that I know how the molecules and chemicals behave from my knowledge of chemistry in 8th grade. I feel like I didn't have any weaknesses in this unit because I understood all of the material that was taught and I have a deeper understanding of how our body uses these macromolecules to function and live. My successes were the labs. The labs that we did in class were the sweetness lab, the enzyme virtual lab, and the cheese lab. The sweetness lab was about tasting different carbohydrates and learning that their sweetness depends on how many "rings" of carbohydrates there were in each molecule. Carbohydrates with one or two rings had a higher sweet content than carbohydrates that had more than two rings, which had a starchy taste. The enzyme virtual lab was about seeing the affects of pH, temperature, substrate and enzyme on a product. The cheese lab was also about seeing the affects of pH, temperature and enzyme on a substrate and product. In the cheese lab, we tested different types of curdling agents, such as chymosin, rennin, buttermilk, and milk. We also tested to see which conditions would curdle the milk faster, such as pH and temperature. Some setbacks were not having proper lab etiquette when we were doing the cheese lab, as many people got pipettes mixed up, possibly contaminating the substances that we were working with.
I learned many things from this unit. I went into deeper understanding of the four macromolecules and I used them in labs to show how they function in real life. I learned about the different structures and functions of the different macromolecules and specific types of each molecule such as monosaccharide, disaccharide, polysaccharide, unsaturated fats, saturated fats, structural proteins, enzymes, DNA, RNA, and ATP. I am a better student than when we started this unit because I learned many new facts and expanded my knowledge in miniature biology.
